Multi-Discipline Automated System
The IDS-iSYS Multi-Discipline Automated System is designed with the flexibility to accommodate unique and challenging assay requirements. The automated system brings testing efficiency and uncompromised quality to specialty immunoassay testing in laboratories of all types and volumes.
The IDS-iSYS Multi-Discipline Automated System is a unique concept that performs to enhance productivity and efficiency, with a high level of quality.

Benefits of IDS iSYS
System Information
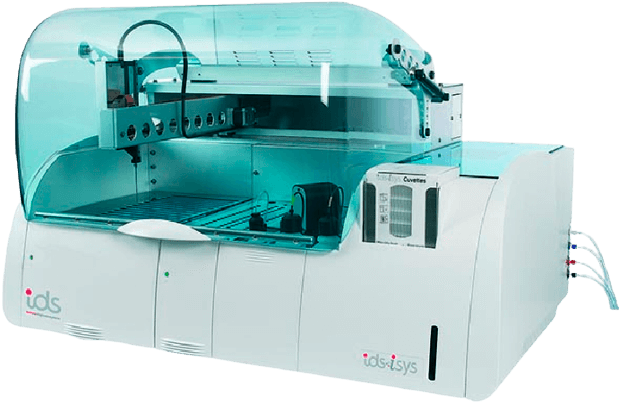
Pipeting area
Liquid level sensing & clot monitoring.
Decontamination process prevents sample carry-over.
Wide range of sample volumes. Sample mixed in cuvette. On-board sample pre-treatment.
Sample area
Up to 120 positions for samples, calibrators and controls (6 racks with 20 positions). Continuous
access, with all types of tube supported.
Reagent area
Up to 15 reagent positions
Continuous loading and automated barcode recognition
Cuvettes
Patented single use cuvette design.
Continuous loading.
Up to 1120 cuvettes on board. Each cuvette box contains 960 cuvettes.
Analytical area
The Modular Analytical Platform (MAP) is a highly reliable assay processing system, with no user
maintenance
Ancillary area
All ancillaries are ready to use and can be continuously loaded during a run. All information is logged for a full traceability.
-
Dimensions
- System: L 105 x H 70 x W 75 cm
- Weight: 103kg
- PC: L 60 x H 40 x W 50cm
Power supply
- Voltage: 100 – 240 V
- Frequency: 50 – 60 Hertz
- Maximum power consumed: 750 VA
Environment
- Sound level: up to 66 dB
- The surrounding temperature must be between 18°C and 30°C
- Emitted heat: up to 530 W (1808 BTU/h or 16.5 cal/s)
- Relative humidity must be within 20 and 80% (non-condensing at 25°C)
-
- Random access
- Time to first results: < 25 minutes (assay dependent)
- Up to 120 tests/hour (assay dependent)
- > 600 results per day (assay dependent)
-
Luminometer
- Wavelength: from 300 to 500 nm
- Linearity: Up to 10 Million RLU
Spectrophotometer
- Spectrophotometer with LED technology
- 12 wavelength interferential filters: 340, 405, 450, 500, 540, 550,
580, 620, 650, 700, 720 and 750 nm - Linearity: Up to 3 Abs
- Bi-chromatic mode availability
- Optic path of cuvettes: 0.8 cm
-
Samples
- 120 positions for samples, calibrators and controls (6 racks with
20 positions) - Serum, plasma, urine, others (saliva…)
- Sampling on Primary Tubes, Secondary Tubes, Micro cups,
pediatric tubes - Default Sample tube library: Primary Tubes: 5 mL (13 x 75 mm),
7 mL (13 x 100 mm), 10 mL (16 x 75 mm) - Secondary Tube: 5 mL (13 x 75 mm)
- Micro cups: 500 mL, 2 mL
- Other sample containers can be defined by the user
- STAT sample
- Automatic reading of barcoded sample tubes (positive identification)
- Barcode types: Code 2/5, EAN/UPC, Code 39, Code 128 and Codabar
- On-board dilution and automatic pre-treatment
Reagent compartment
- Up to 15 different immunoassay cartridges
- Integrated reader for automatic reading of reagent cartridge barcodes
- Onboard storage: 12 – 15 °C
Sample handling
- Single pipetting arm for samples and reagents
- Liquid level detection by capacitance (samples and reagents)
- Clot detection (by pressure transducer)
- Internal and external rinsing between each pipetting of sample
- Possibility of specific rinses using decontaminating solution
- Preheating of sampling (reagents & samples)
- Sample volumes: from 4 μl to 300 μl (assay dependent)
Carousel
- Temperature regulated at 37°C ± 0.5°C
- 90 positions for disposable cuvettes
- 4 Independent washers
Cuvette loader
- Loader for 1 x cube of cuvettes
- 1 x cube contains 960 disposable cuvettes
- 960 cuvettes on board
- During operation, the capacity can be extended to 1120 cuvettes
- Preheated loader
Waste collection
- 10 litres container for liquid waste
- Solid Waste (cuvettes) disposed of in solid waste drawer
- 120 positions for samples, calibrators and controls (6 racks with
-
- IDS-iSYS Liquid System (5 L)
- IDS-iSYS Wash solution (10 L)
- IDS-iSYS Triggers (250 mL each)
- IDS-iSYS D-SORB Solution (2×1 L)
- IDS-iSYS CCS
- IDS-iSYS Cuvettes
- IDS Immunocleaner
-
GUI
- User level management
- Continuous access to programming
- Full traceability: patient results, calibrations, controls…
- Automatic calibration. QC management: up to 4 levels, analysis by Levey-Jennings and Westgard rules (configurable) Lot management of reagents, calibrators and controls by barcodes
- Reagent stability management
- Local storage of 5,000 patient results (including traceability)
- Connection to LIS: bidirectional communication (ASTM protocol), RS-232 or RJ45
Operating system
Minimum configuration required:
- Operating system: Windows 7/10
- Microprocessor: Type Core i5, 3.6 GHz
- Live memory: 4 Gb
- Hard disk: 500 Gb
- Ethernet: 2 independent Ethernet network adaptors
- Ports: USB ports (min 2 one of which at front)
- Input devices: USB Keyboard (country specific), Mouse (optional)
- Screen: 22 inches (touchscreen) monitor, speakers integrated
- Screen resolution: 1920 x 1080 pixels
- Peripherals: Reader-DVD writer, integrated graphics device
Filter Products
Type
Portfolio
Certification
| Assays | Certification | Clinical Area | RUO/IVD |
|---|---|---|---|
| 1,25 VitDXp | CE Marked | CKD-MBDVitamin D | IVD |
| 1,25-Dihydroxy Vitamin D | CE Marked | CKD-MBDSarcoidosisVitamin D | IVD |
| 17-OH Progesterone | CE Marked | Endocrine Reproductive | IVD |
| 25 VitDS | CE Marked | CKD-MBDVitamin D | IVD |
| ACE | CE Marked | Sarcoidosis | IVD |
| AMA (M2) | CE Marked | Liver Disease | IVD |
| ANA Screen | CE Marked | Connective Tissue Disease | IVD |
| Androstenedione | CE Marked | Endocrine Reproductive | IVD |
Type
Portfolio
Certification








